When you pick up a generic inhaler, patch, or injection, you expect it to work just like the brand-name version. But here’s the catch: bioequivalence for these delivery systems isn’t just about matching the dose. It’s about making sure the drug gets to the right place in your body at the right speed - and that’s way harder than it sounds.
Why Bioequivalence Matters More Than You Think
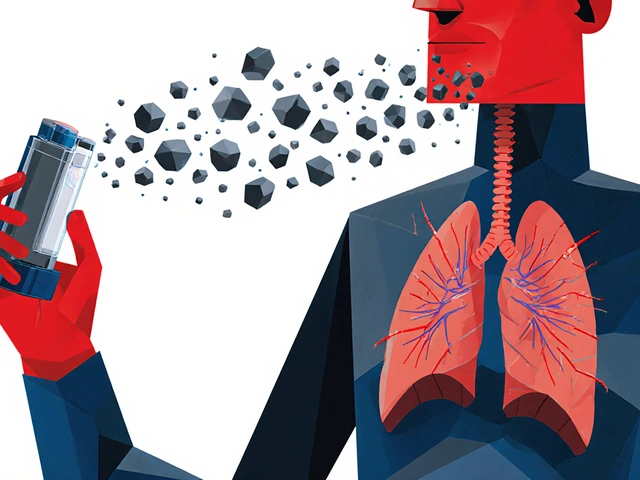
Bioequivalence means two versions of a drug - say, a generic and the original - deliver the same amount of active ingredient to the right spot in your body, at a similar pace. For a pill, that’s straightforward: swallow it, wait for it to dissolve in your stomach, and measure how much shows up in your blood. But for an inhaler, patch, or complex injection? The rules change completely. Take asthma inhalers. If the particles are too big, they hit your throat instead of your lungs. Too small, and they get exhaled before doing any good. The FDA requires that at least 90% of the particles in a generic inhaler fall between 1 and 5 micrometers - the exact size range that reaches deep into the lungs. One study found a generic albuterol inhaler rejected because its plume temperature was 2°C higher than the brand. Sounds tiny? That small difference changed how the drug dispersed in the air, reducing lung delivery by 15%. That’s not bioequivalent. That’s ineffective.Inhalers: It’s Not Just the Drug - It’s the Device
Inhalers come in two main types: metered-dose inhalers (MDIs) and dry powder inhalers (DPIs). Both need to pass a triple test: in vitro, in vivo, and clinical. - In vitro: Labs test particle size, dose uniformity, and how the drug sprays out (plume geometry). If the spray pattern is off, even by a few degrees, it changes where the drug lands in your airway. - In vivo: Patients inhale the drug, and researchers measure either blood levels (for systemic effects) or lung function (like FEV1 - how much air you can force out in one second). For corticosteroid inhalers, blood levels don’t matter much - what matters is whether your lungs open up like they do with the brand. - Clinical: Some regulators now require real-world studies showing the generic performs just as well in treating asthma or COPD over weeks. The approval rate for generic inhalers? Just 38%. That’s the lowest among all complex generics. Why? Because every tiny change - the propellant, the valve, the mouthpiece - affects how the drug behaves. Teva’s generic ProAir RespiClick succeeded because they used scintigraphy imaging to prove identical lung deposition. That’s not just chemistry. That’s physics, engineering, and medicine all in one.Patches: Slow and Steady Wins the Race
Transdermal patches - like nicotine or estrogen patches - are designed to release drug slowly through your skin over hours or days. That’s great for steady symptom control. But it makes bioequivalence tricky. The FDA doesn’t just look at peak blood levels (Cmax). For patches, the total exposure over time (AUC) matters more. The standard 80-125% range still applies, but the timing? It’s stretched out. A patch that releases 10% more drug in the first 6 hours might cause side effects, even if the total dose over 24 hours matches. Testing requires Franz diffusion cells - expensive lab devices that mimic human skin. The patch must release the drug at the same rate at every time point. And it must stick the same way. If the adhesive fails early, the drug stops coming through. If it sticks too well, it can irritate the skin. One generic estrogen patch was pulled after reports of skin burns because the adhesive chemistry was slightly different. The drug was the same. The delivery wasn’t. Approval rate for patches? 52%. Better than inhalers, but still far below the 78% rate for pills. Why? Because skin varies between people - age, hydration, body fat, even where you apply it. A patch that works for one person might not work the same for another. That’s why regulators demand extra proof: residual drug content, skin adhesion strength, and sometimes even skin permeability studies.
Injections: When the Delivery System Is the Drug
Not all injections are the same. A simple saline shot? Easy. But complex injectables - like liposomal doxorubicin, nanoparticle albumin-bound paclitaxel, or low-molecular-weight heparins (like Lovenox)? These aren’t just drugs in a syringe. They’re engineered particles. For these, the FDA requires proof that every physical property matches: particle size (within 10%), surface charge (zeta potential within 5mV), and how the drug leaks out over time (in vitro release profile). For Lovenox, the bioequivalence window is tighter: 90-111% for both Cmax and AUC. Why? Because even a 5% difference in molecular weight distribution can increase bleeding risk. One generic version of Bydureon BCise - a once-weekly injectable for diabetes - was rejected because the auto-injector mechanism delivered the drug slightly slower than the brand. The drug was identical. The device wasn’t. The FDA said no. The sponsor lost $45 million. Approval rate for complex injectables? 58%. Higher than inhalers, but still low. Why? Because manufacturing these isn’t like mixing chemicals. It’s like 3D-printing living particles. One batch might have 10% more nanoparticles than the next. That’s why only big companies with $25-40 million and 3-4 years to spare can even try.Why Are These So Hard and So Expensive?
Let’s compare:- Standard oral generic: $5-10 million to develop. 18-24 months. 78% approval rate.
- Inhaler generic: $25-40 million. 36-48 months. 38% approval rate.
- Complex injectable: $30-50 million. 40+ months. 58% approval rate.

What’s Changing? And What’s Next?
Regulators are catching on. The FDA and EMA now push for a “totality of evidence” approach. That means: in vitro data + physicochemical specs + pharmacokinetics + clinical outcomes - all together. No single test is enough. New tools are helping. Physiologically-based pharmacokinetic (PBPK) modeling lets scientists simulate how a drug behaves in different people - lungs, skin, blood - without testing on hundreds of patients. In 2022, 65% of complex generic submissions included PBPK models. That’s up from 22% in 2018. The biggest threat? “Biocreep.” Imagine five generations of generics, each slightly different. Individually, each passes bioequivalence. But together, the cumulative changes might alter how the drug works. That’s why regulators are now requiring long-term safety data for complex generics - not just short-term blood tests.What Does This Mean for You?
If you’re on a generic inhaler, patch, or injection, you’re getting a product that’s been tested harder than most pills. The system isn’t perfect - approvals are slow, costs are high, and failures are costly. But the goal is clear: safety first, savings second. For patients, that means: if your generic isn’t working like the brand - your asthma isn’t controlled, your patch isn’t sticking, your injection feels different - speak up. Report it. Your feedback helps regulators improve. For the industry, it means innovation isn’t just about new drugs. It’s about making sure the cheapest version works as well as the most expensive one. And that’s worth the fight.What does bioequivalence mean for inhalers?
For inhalers, bioequivalence means the generic must deliver the same amount of drug to the lungs at the same rate as the brand. This requires matching particle size (90% between 1-5 micrometers), dose output, and spray pattern. Testing includes both lab-based in vitro measurements and real patient studies measuring lung function (like FEV1) or drug levels in blood.
Why are generic patches harder to approve than pills?
Patches release drug slowly through the skin, so peak blood levels (Cmax) aren’t as important as total exposure over time (AUC). But skin absorption varies by person, so regulators require exact matching of drug release rates, adhesive strength, and residual drug content. Even a 10% difference in release rate can cause side effects or reduced effectiveness.
Can a generic injection be approved without testing on humans?
Sometimes, but rarely. For complex injectables like liposomes or nanoparticles, regulators require proof of identical physical properties - particle size, surface charge, release profile - before even considering human studies. But for safety-critical drugs like blood thinners, human pharmacokinetic studies are still mandatory. The FDA doesn’t allow substitution based on chemistry alone.
Why do some generic inhalers get rejected even if they deliver the same drug dose?
Because delivery isn’t just about dose - it’s about where the drug lands. If the spray plume is too hot, too fast, or too wide, the drug hits the throat instead of the lungs. One generic was rejected because its plume temperature was 2°C higher than the brand, reducing lung deposition by 15%. The drug was correct. The delivery wasn’t.
Are generic inhalers and patches as safe as brand-name versions?
Yes - if they’re approved. The approval process for complex generics is stricter than for pills. The FDA and EMA require multiple layers of testing: physical properties, lab performance, and often clinical outcomes. If a generic passes all these tests, it’s considered therapeutically equivalent. But because the bar is high, only the most rigorously tested products make it to market.






Noah Fitzsimmons November 20, 2025
Oh wow, so the FDA rejects inhalers because the spray is 2°C warmer? That’s not regulation, that’s a sci-fi movie where the villain controls weather with a vape pen. Next they’ll ban aspirin because the tablet was 0.5mm thicker than the brand and someone’s fingernail got a splinter. 🤡
Eliza Oakes November 22, 2025
Let me guess - the real reason generics fail is because Big Pharma bribes the FDA to keep prices high. They don’t care about lung deposition, they care about your wallet. And don’t even get me started on how ‘bioequivalence’ is just a fancy word for ‘we let them sell it because we ran out of energy to fight.’
Clifford Temple November 24, 2025
USA invented modern medicine. Now we’re letting some cheap lab in India or China make our inhalers with ‘slightly different’ propellants? This isn’t innovation, it’s national surrender. If your generic patch falls off because it was made with glue from a Chinese factory, who’s gonna pay for your ER visit? Not them. Not ever.
Corra Hathaway November 26, 2025
Y’all are overcomplicating this. 😅 If your inhaler doesn’t work, switch back to brand. If your patch falls off, try a new spot. If your injection feels weird, TELL YOUR DOCTOR. We’re not robots - our bodies talk. Listen. 💪 Also, hi! I’m here for you. You got this. ❤️
Shawn Sakura November 28, 2025
It's reaaly hard to make these generics right. Like, the physics of aerosol delivery? It's not just chemistry. It's fluid dynamics, surface tension, even humidity. I've seen labs spend years just tweaking a valve. And yeah, it costs millions. But it's worth it. We need these options. 🙏
Paula Jane Butterfield November 29, 2025
For folks from other countries: this is why the US healthcare system is so broken. We treat medicine like a luxury product, not a right. In Germany, they test generics with real patients across diverse skin types and lung conditions - not just lab simulations. We could do this. We just choose not to.
Simone Wood December 1, 2025
The term 'bioequivalence' is a linguistic sleight of hand. It implies equivalence, but what we’re really measuring is statistical proximity within arbitrary margins. The FDA’s 80-125% window for AUC? That’s a 45% variance. For a drug that treats asthma or cancer? That’s not equivalence - that’s a gamble. And someone’s kid is gonna pay the price.
Swati Jain December 2, 2025
Y’all act like this is new. In India, we’ve been making complex generics since the 90s - insulin, heparin, you name it. The difference? We don’t have $40 million to waste on a single batch. We use clever engineering, not fancy machines. The science is sound - it’s the regulatory greed that’s the problem. 😏
Florian Moser December 2, 2025
Let’s be clear: if a generic inhaler delivers 95% of the drug to the lungs and matches the pharmacokinetic profile, it’s therapeutically equivalent. The 2°C plume temperature issue? That’s a manufacturing control problem, not a bioequivalence failure. We should be focusing on outcomes, not physics minutiae. This over-engineering is slowing access to life-saving meds.
jim cerqua December 3, 2025
THIS IS A SCAM. I’ve been on a generic patch for three months. My skin burns. My doctor says ‘it’s fine.’ But I know. I’ve seen the videos. The FDA lets these things through because they’re scared of lawsuits from big pharma. And now? My insurance won’t cover the brand anymore. So I’m stuck. This isn’t healthcare. This is corporate warfare. And I’m the casualty.
Donald Frantz December 4, 2025
What’s the data on long-term outcomes for approved generics vs. brand? Are hospitalization rates the same? Are ER visits for asthma attacks lower? If we’re talking bioequivalence, we need real-world clinical data - not just lab measurements. PBPK modeling is great, but it’s still a simulation. Real people breathe real air. Real skin reacts to real adhesives.
Sammy Williams December 4, 2025
My uncle’s on a generic COPD inhaler. Works fine. He’s 72. Doesn’t care if it’s made in China or Ohio. He just wants it cheap. Maybe we’re over-engineering this for people who don’t need it to be perfect. Sometimes ‘good enough’ is actually good.
Julia Strothers December 5, 2025
Remember when the FDA approved a generic heparin in 2008 and 100 people died? That’s what happens when you let ‘bioequivalence’ be a checkbox. Now they’re using PBPK models and ‘totality of evidence’? That’s just PR. The same people are still in charge. The same labs. The same silence. This isn’t science - it’s a controlled demolition of patient safety. And they’re calling it progress.