Home » Allergy » Telfast
Telfast
Brand Name: Telfast, Allegra
Generic Name: Fexofenadine hydrochloride
Fexofenadine hydrochloride (brand names include Allegra and Telfast) is an antihistamine drug used in the treatment of hayfever and similar allergy symptoms.
DESCRIPTION
Fexofenadine hydrochloride, the active ingredient of TELFAST, is a histamine H1- receptor. It has the following chemical structure.
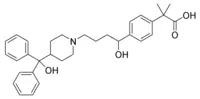
The molecular weight is 538.13 and the empirical formula is C32H39NO4HCI.
Fexofenadine hydrochloride is a white to off-white crystalline powder. It is freely soluble in methanol and ethanol, slightly soluble in chloroform and water, and insoluble in hexane Fexofenadine hydrochloride is a racemate and exists as a zwitterion in aqueous media at
physiological pH.
TELFAST is formulated as a tablet for oral administration. Each tablet contains 30 mg or 60 mg fexofenadine hydrochloride (depending on the dosage strength) and the following excipients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and pregelatinized starch. The aqueous tablet film coating is made from hydroxypropyl methylcellulose, iron oxide blends, polyethylene glycol, povidone, silicone dioxide, and titanium dioxide.
CLINICAL PHARMACOLOGY
Mechanism of Action
Fexofenadine hydrochloride is an antihistamine with selective peripheral H1-receptor antagonist activity. Fexofenadine inhibited antigen-induced bronchospasm in sensitized guinea pigs and histamine release from peritoneal mast cells in rats. In laboratory animals, no anticholinergic or alpha1-adrenergic-receptor blocking effects were observed. Moreover, no sedative or other central nervous system effects were observed. Radiolabeled tissue distribution studies in rats indicated that fexofenadine does not cross the blood-brain barrier.
Pharmacokinetics
Absorption:
Fexofenadine hydrochloride was rapidly absorbed following oral administration of a single dose of two 60 mg capsules to healthy male volunteers with a mean time to maximum plasma concentration occurring at 2.6 hours post-dose. After administration of a single 60-mg dose as an oral solution to healthy subjects, the mean plasma concentration was 209 ng/mL. Mean steady-state peak plasma concentrations of 286 ng/mL were observed when healthy volunteers were administered multiple doses of fexofenadine hydrochloride (60 mg oral solution every 12 hours for 10 doses). Although the absolute bioavailability of fexofenadine hydrochloride capsules is unknown, the capsules are bioequivalent to an oral solution. In addition, tablets are also bioequivalent to capsules. Fexofenadine hydrochloride pharmacokinetics are linear for oral doses up to a total daily dose of 240 mg (120 mg twice daily).
Distribution:
Fexofenadine hydrochloride is 60% to 70% bound to plasma proteins, primarily albumin and alpha1acid glycoprotein.
Elimination:
The mean elimination half-life of fexofenadine was 14.4 hours following administration of 60 mg. twice daily, in normal volunteers.
Human mass balance studies documented a recovery of approximately 80% and 11% of the [14C] fexofenadine hydrochloride dose in the feces and urine, respectively. Because the absolute bioavailability of fexofenadine hydrochloride has not been established, it is unknown if the fecal component represents unabsorbed drug or the result of biliary excretion.
Fexofenadine clearance in children aged 6 to 11 years is approximately 40% slower than in adults. Therefore, a dose of 30 mg BID was determined to provide plasma levels (AUC and Cmax) in children which were comparable to plasma levels achieved in adults following 120 mg once daily.
Metabolism:
Approximately 5% of the total oral dose was metabolized.
The pharmacokinetics of fexofenadine hydrochloride in seasonal allergic rhinitis and chronic idiopathic urticaria patients were similar to those in healthy subjects: Peak fexofenadine plasma concentrations were similar between adolescent (12-16 years of age) and adult patients.
Special Populations
Special population pnarmacokinetia (for geriatric subjects, renal and hepatic impairment), obtained after a single dose of 80 mg fexofenadine hydrochloride, were compared to those for normal subjects from a separate study of similar design. While subject weights were relatively uniform between studies, these adult special population patients were substantially older than the healthy, young volunteers .Thus, an age effect may be confounding the pharmacokinetic differences observed in some of the special populations.
Geriatric Subjects.
In older subjects (>65 years old), peak plasma levels of fexofenadine were 99% greater than those observed in normal volunteers (
Pediatrics patients.
Cross study comparisons indicated that fexofenadine hydrochloride area under the curve (AUC) following oral administration of a 60 mg dose to 7-12 year old pediatric allergic rhinitis patients was 56% greater compared to healthy adult subjects given the same dose. Plasma exposure in pediatric patients given 30 mg fexofenadine hydrochloride is comparable to adults given 60 mg.
Renal Impairment.
In patients with mild to moderate (creatinine clearance 41-80mL/min) and severe (creatinine clearance 11-40mL/min) renal impairment, peak plasma levels of fexofenadine were 87% and 111% greater, respectively, and mean elimination half-lives were 59% and 72% longer, respectively, than observed in normal volunteers. Peak plasma levels in patients on dialysis (creatinine clearance
Hepatic Impairment
The pharmacokinetics of fexofenadine hydrochloride in patients with hepatic disease did not differ substantially from that observed in healthy subjects.
Effect of Gender.
Across several trials, no clinically significant gender-related differences were observed in the pharmakionetics of fexofenadine hydrochloride.
Pharmacodynamics
Wheal and Flare.
Human histamine skin wheal and flare studies conducted in adults following single and twice daily doses of 20 mg and 40 mg fexofenadine hydrochloride demonstrated that the drug exhibits an antihistamine effect by 1 hour, achieves maximum effect at 2 to 3 hours, and an effect is still seen at 12 hours. There was no evidence of tolerance to these effects after 28 days of dosing.
In children aged 6-11 years, fexofenadine hydrochloride suppressed histamine-induced wheal and flare to a similar extent to that observed in adults. Histamine skin wheal and flare studies in pediatric patients showed that following a single dose of 30 or 60 mg, antihistamine effect was observed at 1 hour and reached a maximum by 3 hours. Greater than 49% inhibition of wheal area, and 74% inhibition of flare area were maintained for 8 hours following the 30 and 60 mg dose.
Effects on QTc.
In dogs, (10 mg/kg/day, orally for 5 days) and rabbits (10 mg/kg intravenously over one hour) fexofenadine did not prolong QTc at plasma concentrations that were at least 28 and 63 times, respectively, the therapeutic plasma concentrations in man (based on a 60 mg twice daily fexofenadine hydrochloride dose). No effect was observed on calcium channel current, delayed potassium channel current or action potential duration in guinea pig myocytes. Sodium current in rat neonatal myocytes, or on the delayed rectifier potassium channel cloned from human heart at
concentrations up to 1x10-5 M of fexofenadine. This concentration was at least 32 times the
therapeutic plasma concentration in man (based on a 60 mg twice daily fexofenadine hydrochloride dose.
No statistically significant increase in mean QTc interval compared to placebo was observed in 714 seasonal allergic rhinitis patients given fexofenadine hydrochloride capsules in doses of 60 mg to-240 mg twice daily for two weeks or in 40 healthy volunteers given fexofenadine hydrochloride as an oral solution at doses up to 400 mg twice daily for 6 days Pediatric patients from two placebo controlled trials (n=855) treated with up to 60 mg fexofenadine hydrochloride twice daily demonstrated no significant treatment or dose-related increases in QTc.
Clinical Studies
Adults
In three, 2-week, multi-center, randomized, double blind, placebo-controlled trials in patients 12 to 68 years of age with seasonal allergic rhinitis (n = 1634), fexofenadine hydrochloride 60 mg twice daily significantly reduced total symptom scores (the sum of the individual scores for sneezing, rhinorrhea, itchy nose/palate/throat, itchy/watery/red eyes) compared to placebo. Statistically significant reductions in symptom scores were observed following the first 60-mg dose, with the effect maintained throughout the 12-hour interval. In these studies, there was no additional reduction in total symptom scores with higher doses of fexofenadine hydrochloride up to 240 mg twice daily. Although the number of patients in some of the subgroups was small., there were no significant differences in the effect of fexofenadine hydrochloride across subgroups of patients defined by gender, age and race. Onset of action for reduction in total symptom scores, excluding nasal congestion, was observed at 60 minutes compared to placebo following a single 60-mg i fexofenadine hydrochloride dose administered to patients with seasonal allergic rhinitis who were exposed to ragweed pollen in an environmental exposure unit.
Pediatrics
Two 2-week multicenter, randomized, placebo-controlled, double-blind trials in 877 pediatric patients 6 to 11 years of age with seasonal allergic rhinitis were conducted at doses at 15, 30 and 60 mg twice daily. In one of these two studies, conducted in 411 pediatric patients aft three doses of fexofenadine hydrochloride significantly reduced total symptom scores (the sum of the individual scores for sneezing, rhinorrhea, itchy nose/paIate/throat, itchy/ watery/red eyes) compared to placebo, however a dose response relationship was not seen. The 60 mg twice daily dose did not provide any additional benefit over the 30 mg twice daily dose. Furthermore, exposure m pediatric patients given 30 mg fexofenadine hydrochloride is comparable to adults given 60 mg (see CLINICAL PHARMACOLOGY.)
INDICATIONS AND USAGE
TELFAST is indicated for the relief of symptoms associated with allergic rhinitis in adults and children 6 years of age and older. Symptoms treated effectively include sneezing, rhinorrhea, itchy nose / palate / throat, itchy / watery / red eyes.
TELFAST is indicated for the relief of symptoms associated trim chronic idiopathic urticaria in adults and children 6 years of age and older.
CONTRAINDICATIONS
TELFAST is contraindicated in patients with known hypersensitivity to any of its ingredients.
PRECAUTIONS
Drug Interaction with Erythromycin and Ketoconazole.
Fexofenadine hydrochloride has been shown to exhibit minimal (ca. 5%) metabolism. However, co-administration of fexofenadine hydrochloride with ketoconazole and erythromycin led to increased plasma levels of fexofenadine hydrochloride. Fexofenadine hydrochloride had no effect on the pharmacokinetics of erythromycin and ketoconazole. In two separate studies, fexofenadine hydrochloride 120 mg twice daily (two times the recommended twice daily dose) was co-administered with erythromycin 500 mg every 8 hours or ketoconazole 400 mg once daily under steady-state conditions to normal, healthy volunteers (n = 24, each study). No differences in adverse events or QTc interval were observed when patients were administered fexofenadine hydrochloride alone or in combination with erythromycin or ketoconazole.
Buy Telfast, Fexofenadine, Allegra
More Fexofenadine info
Fexofenadine
Fexofenadine hydrochloride
Fexofenadine MedlinePlus
|



