When you pick up a prescription, you might not realize the pharmacist just swapped your brand-name drug for a cheaper generic. That’s not a mistake-it’s legal. But pharmacy substitution laws aren’t the same everywhere. In one state, the pharmacist can switch your medication without telling you. In another, they need your signed permission. And in some cases, they can’t substitute at all. If you’re on a long-term medication, especially for something like epilepsy, thyroid disease, or an autoimmune condition, this isn’t just paperwork-it’s your health.
What Exactly Is Pharmacy Substitution?
Pharmacy substitution means a pharmacist gives you a generic version of your prescription instead of the brand-name drug your doctor wrote. Generic drugs have the same active ingredient, dose, and effect as the brand-name version. They’re tested by the FDA to be just as safe and effective. The big difference? Price. Generics cost 80-85% less. That’s why pharmacies and insurers push for substitution-it saves billions each year. But not all drugs are created equal. For most medications, switching is fine. For others-like warfarin, phenytoin, or levothyroxine-tiny differences in how the drug is made can cause big problems. These are called narrow therapeutic index (NTI) drugs. A small change in blood levels can lead to a seizure, a blood clot, or a thyroid crisis. That’s why some states block substitution for these drugs entirely.How State Laws Differ
All 50 U.S. states and Washington, D.C. have laws about substitution. But they don’t agree on much else. In 19 states, pharmacists must substitute generics unless the doctor says no. That means if your prescription says "Lipitor," and a generic atorvastatin is available, the pharmacist gives you the generic by default. You won’t be asked. You might not even know. In the other 31 states, substitution is optional. The pharmacist can switch it, but only if they choose to. Some do. Some don’t. It depends on the pharmacy, the pharmacist, or even how busy the day is. Then there are the restrictions. Hawaii won’t let pharmacists substitute antiepileptic drugs without both the doctor and patient saying yes. Oklahoma says pharmacists can’t substitute unless the patient or prescriber gives direct permission. Kentucky has a list of 12 drugs that can’t be swapped at all-even if they’re technically generics.What About Biosimilars?
Biosimilars are the next wave. These aren’t simple chemical copies like generics-they’re complex biological products made from living cells. Think drugs for rheumatoid arthritis, psoriasis, or cancer. The FDA has approved 38 biosimilars so far, but only 10 are officially labeled "interchangeable." That means they’ve passed extra tests to prove they can be swapped like a generic. States treat interchangeable biosimilars like a whole different game. Forty-five states have stricter rules for them than for regular generics. Thirty-seven states require the pharmacist to notify your doctor within a few days after swapping. Twelve states require the doctor to approve the switch before it happens. Fifteen states won’t let you substitute if the biosimilar costs more than the original-even if your insurance covers it. In California, since January 2023, pharmacists must give you written notice before switching a biosimilar. In New York, they can now substitute certain biosimilars without waiting for the doctor’s okay. But in Alabama and Mississippi, the rules are minimal-just log the swap and move on.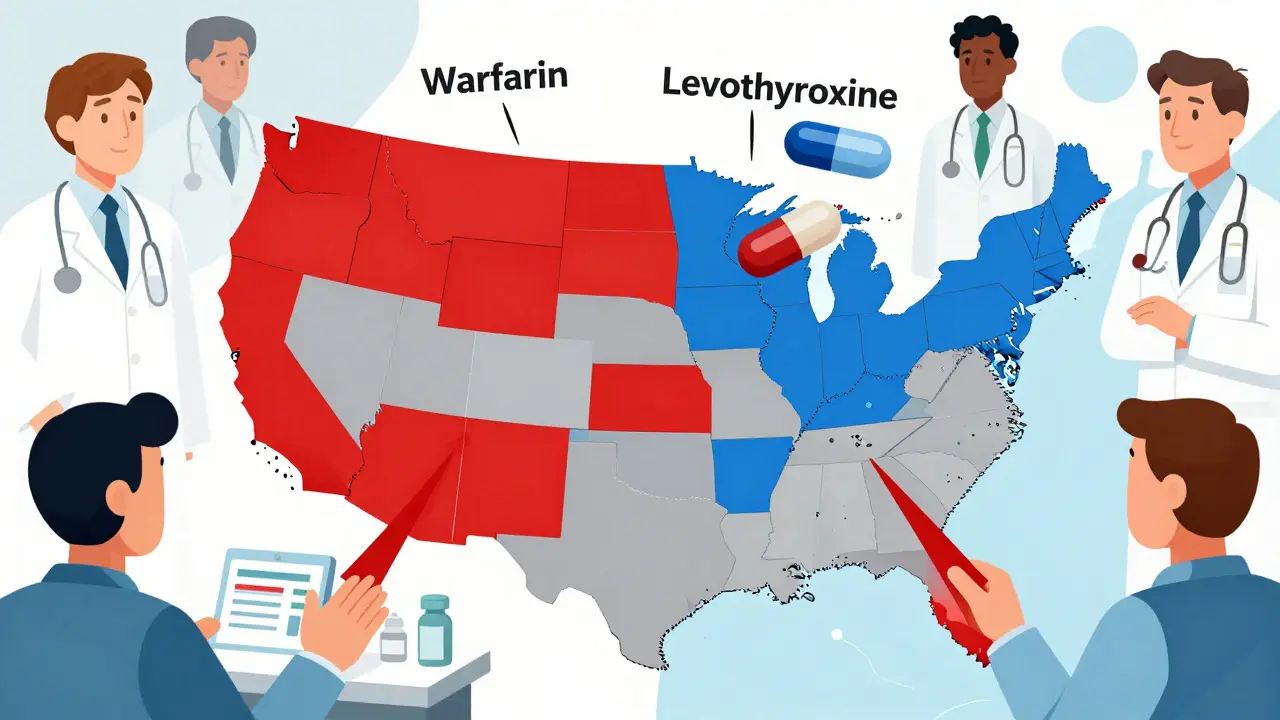
What You Can Do as a Patient
You have rights-even if the pharmacist doesn’t tell you about them. First, you can always say no. In every state, you can refuse a substitution. But in 24 states, the pharmacist isn’t required to tell you that you have that right. So if you’re handed a different pill and you’re not sure, ask: "Is this the same as what my doctor prescribed?" If it’s not, you can ask for the original. Second, check your prescription. If your doctor wrote "dispense as written" or "do not substitute," the pharmacist legally can’t switch it. But here’s the catch: in 18 states, the doctor must write a reason for that note. If they just scribble "no substitution," it might get ignored. Third, know your drug. If you’re on a narrow therapeutic index medication-warfarin, levothyroxine, phenytoin, cyclosporine, or digoxin-ask your pharmacist if substitution is allowed. Even if your state allows it, your doctor might want to keep you on the same brand. Studies show states with NTI restrictions have 18% fewer adverse events.What Pharmacists Need to Know
Pharmacists are on the front line. They’re expected to know their state’s law, track which drugs are interchangeable, document every swap, and notify patients and prescribers-all while managing a busy pharmacy. In 24 states, pharmacists have no legal protection if something goes wrong after a substitution. That means if a patient has a bad reaction, even if the pharmacist followed every rule, they could still be sued. That’s why many pharmacists hesitate to substitute, even when it’s allowed. The good news? Most states require pharmacists to keep records of substitutions for at least two years. That’s not just for legal protection-it’s for patient safety. If you switch back and forth between brands and generics, your doctor needs to know what you’ve actually been taking.How to Find Your State’s Rules
There’s no national database. You have to look it up yourself. Start with your state’s Board of Pharmacy website. Most have a section on drug substitution laws. If you can’t find it, call them. Ask: "What are the rules for generic and biosimilar substitution in my state?" You can also check the National Association of Boards of Pharmacy’s model legislation page. Twenty-two states have used their template since 2020, so their rules are starting to look more alike. Look for keywords like "shall," "may," or "must"-those tell you if substitution is required or optional. For biosimilars, the FDA’s Purple Book lists which drugs are interchangeable. If your drug isn’t on that list, substitution isn’t legally allowed-even if the pharmacy says it is.Why This Matters for Your Health
It’s easy to think, "It’s just a generic. It’s the same thing." But for some people, it’s not. A 2009 study found that in states requiring patient consent before substitution, generic use for simvastatin dropped by 25%. That sounds bad-until you realize those same states saw fewer hospital visits and emergency trips because patients were more aware of what they were taking. When patients know they’ve been switched, they’re more likely to notice side effects. They’re more likely to tell their doctor. They’re more likely to stick with the treatment. The goal of substitution laws is to save money. And they do. Generics save the U.S. system $313 billion a year. But saving money shouldn’t mean risking safety. The best laws balance both.What’s Changing Soon
More biosimilars are getting interchangeable status every year. By 2030, experts predict they could make up 70% of the biologics market. That means more substitutions. More confusion. More need for clear rules. States are slowly moving toward standardization. The National Association of Boards of Pharmacy is pushing for consistent notification times, documentation rules, and patient rights across all states. But until then, you can’t assume anything. If you travel, move, or switch pharmacies, your substitution rules change. What worked in Texas might not fly in Maine. Always double-check.Can my pharmacist substitute my brand-name drug without telling me?
In 31 states and Washington, D.C., pharmacists can substitute generics without telling you. In 7 states, they must get your explicit consent before switching. In the rest, they must notify you after the fact. Always ask if you’re unsure-your pharmacist is required to answer.
Can I refuse a generic drug even if my state allows substitution?
Yes. In every state, you have the right to refuse a substitution and ask for the brand-name drug. You may have to pay more out of pocket, but you can’t be forced to take a different medication. Tell the pharmacist clearly: "I want the exact drug my doctor prescribed."
Are biosimilars the same as generics?
No. Generics are exact chemical copies of small-molecule drugs. Biosimilars are highly similar to complex biological drugs made from living cells, but not identical. Only 10 biosimilars in the U.S. have FDA approval as "interchangeable," meaning they can be swapped like generics. The rest require special rules.
What if my insurance only covers the generic?
Insurance often pushes for generics to cut costs. But if your doctor says you need the brand, they can write "dispense as written" on the prescription. In 28 states, they must also give a reason. If your insurer denies coverage, ask your doctor to appeal or file a prior authorization request.
How do I know if my drug is a narrow therapeutic index (NTI) medication?
Common NTI drugs include warfarin (blood thinner), levothyroxine (thyroid), phenytoin (seizure), cyclosporine (transplant), and digoxin (heart). If you’re on one of these, ask your pharmacist or doctor if substitution is allowed in your state. Many states ban it for these drugs. Don’t assume it’s safe.






jay patel February 3, 2026
man i just got switched from my brand levothyroxine to some generic last week and felt like a zombie for two weeks. my doc never said anything about it and the pharmacist just handed me a different bottle. now i check every single pill like a paranoid squirrel. why do they even let this happen without telling us? its not like we’re swapping out toilet paper here.
also side note: if you’re on warfarin, DO NOT let them switch you. i had a cousin who almost bled out because some pharmacist thought ‘it’s the same thing.’ it’s not. it’s not.
and yeah i know generics save money but if i’m gonna die because of a 50-cent savings, i’d rather pay the extra bucks and sleep at night.
Ansley Mayson February 3, 2026
stop whining. generics are FDA approved. if you can’t handle a 10% difference in inactive ingredients you’re not fit to live in a modern society. this isn’t 1987. we have science now.
also your ‘narrow therapeutic index’ nonsense is just pharma fearmongering to keep prices high. grow up.
Hannah Gliane February 5, 2026
OH MY GOD I JUST REALIZED MY PHARMACIST SWITCHED MY CICLOSPORINE LAST MONTH 😭😭😭
and now i’m having tremors and my hair is falling out and i didn’t even know i could SAY NO??
why does no one tell you this?? i feel like a lab rat.
also why do pharmacists act like they’re doing us a favor?? they’re not heroes. they’re just following a script written by insurance companies who don’t care if we live or die. 😤
ps: i’m now demanding my brand name and paying out of pocket. no more ‘it’s the same thing’ bullshit.
Murarikar Satishwar February 5, 2026
the key here is awareness and documentation. if you’re on an NTI drug, always ask your pharmacist if substitution is allowed under your state law. most of them will tell you honestly if you ask politely.
also, keep a simple log: date, drug name, brand/generic, pharmacy. even a note in your phone works. this helps your doctor spot patterns if you start feeling off.
and yes, you can refuse. just say ‘i’d like the exact medication my doctor prescribed.’ no explanation needed. they’re legally required to honor that.
also, check your state’s board of pharmacy website. most have PDFs of the exact statutes. it’s not hard to find. just search ‘[your state] board of pharmacy substitution laws’.
Ellie Norris February 6, 2026
oh my god i had no idea about biosimilars being different from generics! i thought they were just fancy generics.
i’m on a biosimilar for rheumatoid arthritis and my pharmacist just swapped it last month and didn’t say a word. i’ve been feeling awful since. i’m calling my doctor tomorrow.
also, can we talk about how ridiculous it is that california requires written notice but alabama just logs it and moves on?? we’re in the same country. this is chaos.
ps: i just googled my state’s rules and found a whole page. it’s actually kinda helpful. try it!
Marc Durocher February 7, 2026
so i used to work at a pharmacy and let me tell you, pharmacists are drowning. they’re juggling 100 scripts an hour, insurance denials, angry patients, and now they’re supposed to know every state’s weird substitution rule AND document everything?
most of them just do what’s easiest. if the generic is in stock and the insurance likes it, they grab it. no malice. just exhaustion.
and yeah, sometimes they forget to tell you. not because they’re evil, but because they’re human and overworked.
so ask. just ask. ‘is this the same as my last script?’ it takes 3 seconds. saves your life.
clarissa sulio February 8, 2026
if you’re paying for brand name drugs in 2025 you’re a sucker. the system works. generics are safe. stop being dramatic.
we’re not in europe. we don’t have free healthcare. if you can’t afford your meds, get a job or move to canada.
also stop blaming pharmacists. they’re just following the rules you all voted for.
Bob Hynes February 9, 2026
in canada we don’t even have this problem. if you want brand, you pay for it. if you want generic, you get it. no drama. no legal maze. no ‘did they tell you?’
but here? it’s like a game of russian roulette with your thyroid levels.
i moved from toronto to texas last year and had a seizure because my phenytoin got switched and no one warned me. i’m lucky i didn’t die.
now i carry a laminated card in my wallet that says: ‘DO NOT SUBSTITUTE - NTI DRUG - SEE DOCTOR.’
if you’re on one of these meds, do the same. it’s not paranoia. it’s survival.
Monica Slypig February 9, 2026
you people are ridiculous. this is why america is falling apart. everyone wants everything handed to them on a silver platter while complaining about the cost.
you think your life is so special? your thyroid is so fragile? get over it.
if you can’t handle a generic, maybe you shouldn’t be on medication at all. just sayin’.
Becky M. February 10, 2026
my mom is on levothyroxine and she’s been on the same brand for 12 years. her doctor wrote ‘do not substitute’ on every script. she never knew she had to ask.
last month the pharmacist gave her the generic and she didn’t notice until her heart started racing. she called me screaming.
we called the doctor, he called the pharmacy, they apologized and switched her back.
but why did it take her having a panic attack to realize something changed?
i’m starting a spreadsheet for all our meds now. i’m not waiting for another scare.
Eli Kiseop February 11, 2026
so if i’m on warfarin and they switch me and i dont notice until i start bruising everywhere is that the pharmacists fault or mine for not asking
also why is this so complicated
why cant it just be simple
like if its a generic then just say its a generic
larry keenan February 12, 2026
the regulatory fragmentation across state lines represents a significant systemic inefficiency in pharmaceutical distribution. while cost containment objectives are laudable, the absence of harmonized substitution protocols introduces clinically significant variability in therapeutic outcomes, particularly for narrow therapeutic index agents.
the current model places undue cognitive burden on both patients and providers, and the lack of standardized documentation protocols compromises longitudinal safety monitoring.
recommendation: federal oversight through FDA guidance with mandatory electronic notification to EHR systems upon substitution, coupled with state-level NTI drug registries.
Nick Flake February 13, 2026
we treat medicine like a commodity. but your body isn’t a vending machine. you don’t get the same effect from two different brands of insulin or thyroid meds just because they have the same active ingredient.
it’s like swapping out your favorite guitar for a knockoff that looks identical but sounds off. you might not notice at first. but after a few weeks, your whole song is wrong.
we need to stop pretending biology is a spreadsheet. your health isn’t a line item.
and if the system doesn’t care enough to tell you what you’re getting, maybe we need to change the system.
not just you asking. not just doctors fighting. we need a law that says: ‘if you change it, you tell them.’
simple. human. right.
❤️