Generic Drug Stocking: What Pharmacies Need to Know About Supply, Safety, and Savings
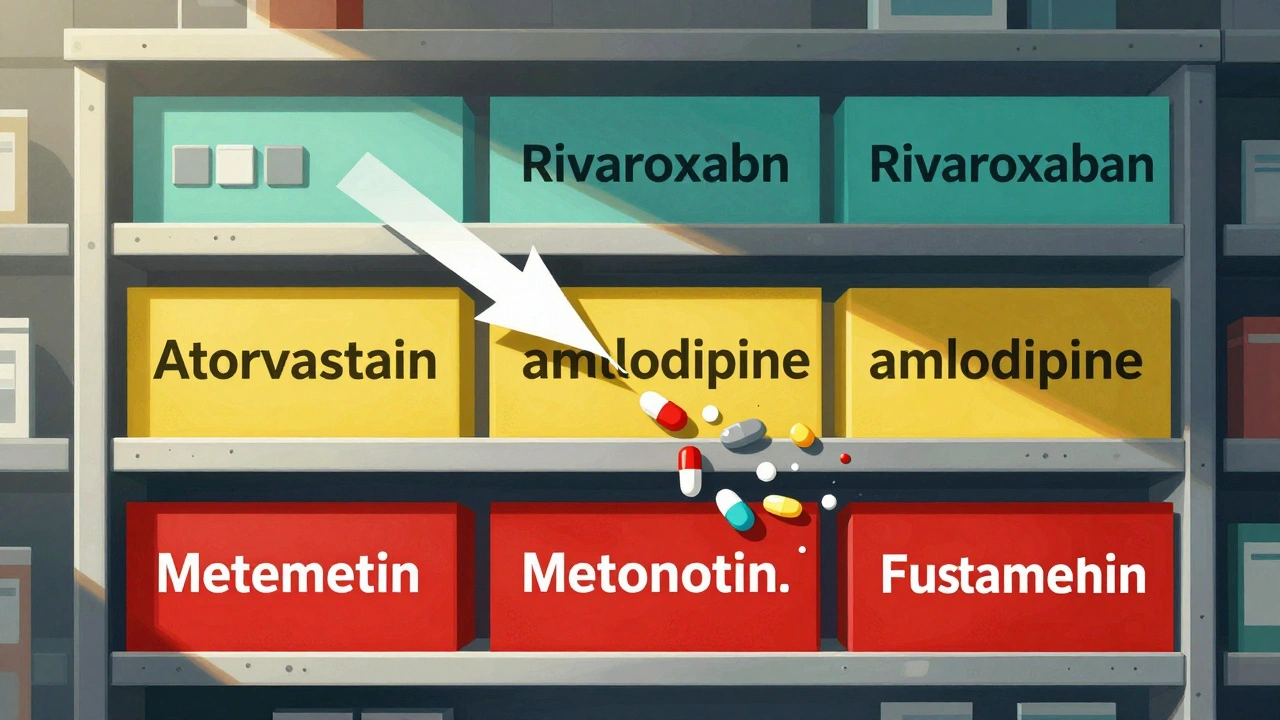
When it comes to generic drug stocking, the process of sourcing, storing, and dispensing FDA-approved alternatives to brand-name medications. Also known as generic medication inventory, it’s not just about filling shelves with cheaper pills—it’s about keeping patients safe, compliant, and treated without unnecessary cost. Every bottle you stock carries legal, clinical, and financial weight. The FDA requires generics to match brand-name drugs in active ingredients, strength, dosage form, and bioequivalence, how closely a generic drug performs in the body compared to its brand-name counterpart. That means if a patient switches from Lipitor to atorvastatin, their body should respond the same way. But stocking isn’t done once the paperwork is signed. You need to track stability, expiration dates, and supplier reliability—because even a tiny degradation can turn a safe drug into a risk.
Then there’s the drug supply chain, the network of manufacturers, distributors, and pharmacies that move medications from production to patient. It’s fragile. When one overseas factory faces a quality issue, it can ripple through dozens of pharmacies. You’ve seen it: a popular generic disappears, patients panic, doctors scramble. That’s why smart stocking means having backup suppliers, understanding which generics are made where, and knowing which ones have a history of recalls. Not all generics are created equal in the eyes of regulators or patients. Some are complex—like inhalers or injectables—where bioequivalence, how closely a generic drug performs in the body compared to its brand-name counterpart isn’t just about blood levels. It’s about how the drug is delivered. A faulty inhaler design can mean a patient doesn’t get the full dose, even if the chemical is identical.
And let’s not forget the human side. Patients trust you to give them the right med at the right price. But when they notice a change in pill color, size, or even how they feel after switching, they come to you—not the manufacturer. That’s why your stocking decisions must be backed by clear documentation, staff training, and open communication. You’re not just moving inventory—you’re managing expectations. The generic drug stocking process isn’t just a cost-saving exercise. It’s a precision operation where legal obligations, patient safety, and supply chain realities collide. What you find below are real-world insights from pharmacists, regulators, and manufacturers who’ve lived through recalls, shortages, and the quiet victories of keeping essential meds on the shelf. Whether you’re managing a small clinic or a large chain, these posts will show you how to stock smarter, not harder.
Pharmacy Inventory Management: Generic Stocking Strategies That Cut Costs and Prevent Stockouts
Learn how to manage generic medication inventory with proven strategies that cut costs, prevent stockouts, and improve pharmacy profitability. Real-world methods for stocking, software, and staff training.